Important Safety Information
WARNINGS AND PRECAUTIONS
Effusion and Edema
Serious effusion and edema occurred in patients treated with ZYNLONTA®. Grade 3 edema occurred in 3% (primarily peripheral edema or ascites) and Grade 3 pleural effusion occurred in 3% and Grade 3 or 4 pericardial effusion occurred in 1%.
Monitor patients for new or worsening edema or effusions. Withhold ZYNLONTA® for Grade 2 or greater edema or effusion until the toxicity resolves. Consider diagnostic imaging in patients who develop symptoms of pleural effusion or pericardial effusion, such as new or worsened dyspnea, chest pain, and/or ascites such as swelling in the abdomen and bloating. Institute appropriate medical management for edema or effusions.
Myelosuppression
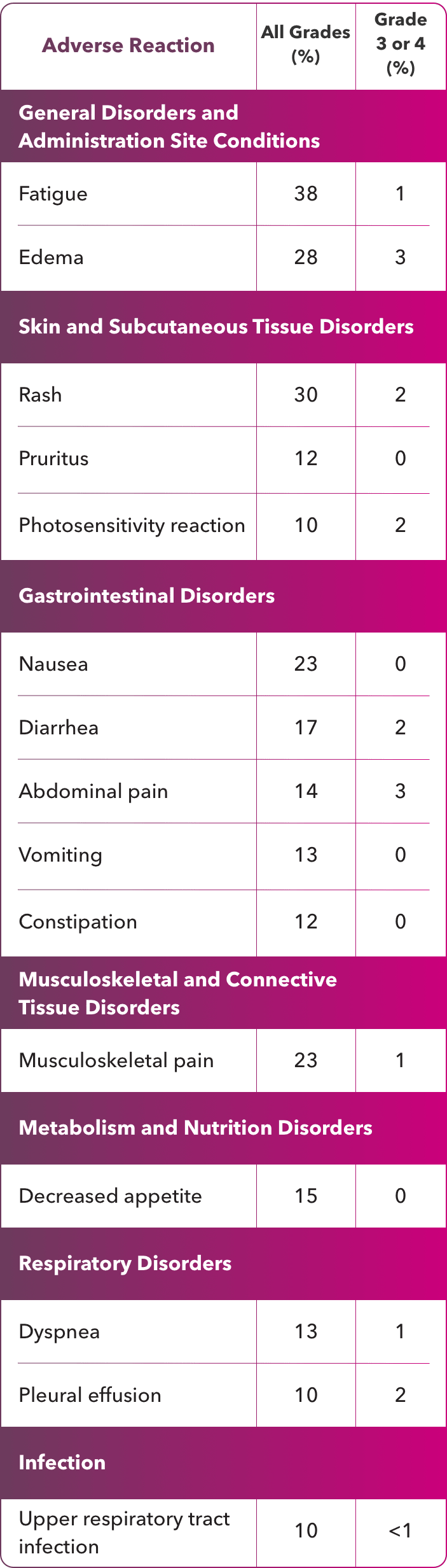
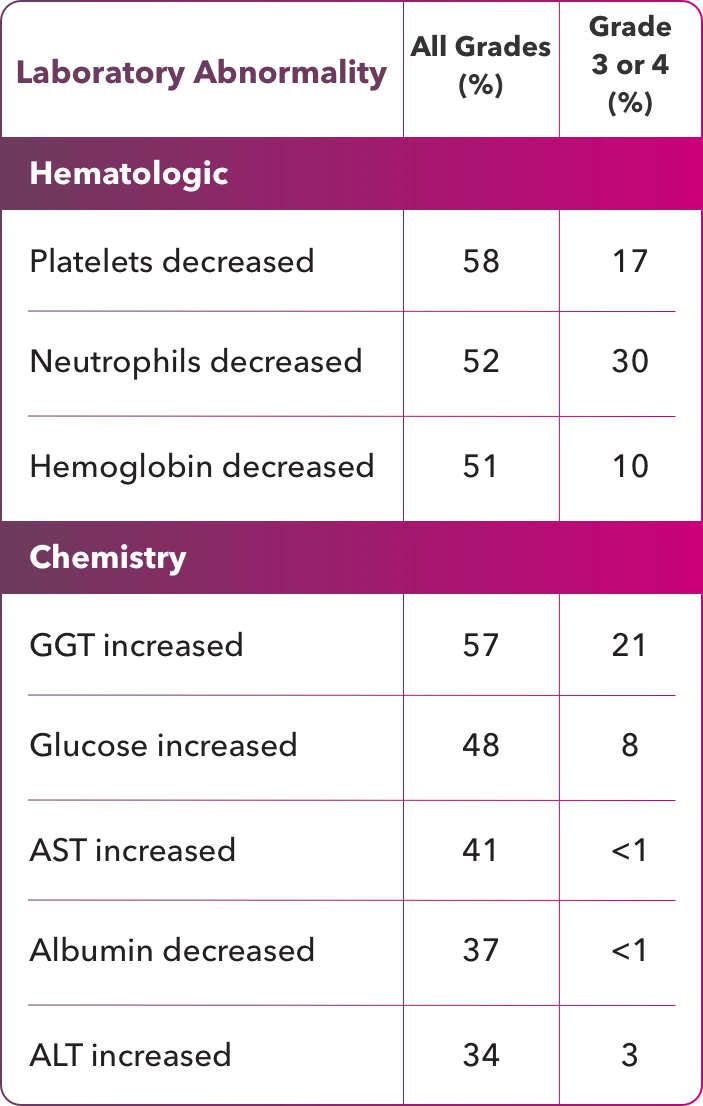
Treatment with ZYNLONTA® can cause serious or severe myelosuppression, including neutropenia, thrombocytopenia, and anemia. Grade 3 or 4 neutropenia occurred in 32%, thrombocytopenia in 20%, and anemia in 12% of patients. Grade 4 neutropenia occurred in 21% and thrombocytopenia in 7% of patients. Febrile neutropenia occurred in 3%.
Monitor complete blood counts throughout treatment. Cytopenias may require interruption, dose reduction, or discontinuation of ZYNLONTA®. Consider prophylactic granulocyte colony-stimulating factor administration as applicable.
Infections
Fatal and serious infections, including opportunistic infections, occurred in patients treated with ZYNLONTA®. Grade 3 or higher infections occurred in 10% of patients, with fatal infections occurring in 2%. The most frequent Grade ≥3 infections included sepsis and pneumonia.
Monitor for any new or worsening signs or symptoms consistent with infection. For Grade 3 or 4 infection, withhold ZYNLONTA® until infection has resolved.
Cutaneous Reactions
Serious cutaneous reactions occurred in patients treated with ZYNLONTA®. Grade 3 cutaneous reactions occurred in 4% and included photosensitivity reaction, rash (including exfoliative and maculo-papular), and erythema.
Monitor patients for new or worsening cutaneous reactions, including photosensitivity reactions. Withhold ZYNLONTA® for severe (Grade 3) cutaneous reactions until resolution. Advise patients to minimize or avoid exposure to direct natural or artificial sunlight including exposure through glass windows. Instruct patients to protect skin from exposure to sunlight by wearing sun-protective clothing and/or the use of sunscreen products. If a skin reaction or rash develops, dermatologic consultation should be considered.
Embryo-Fetal Toxicity
Based on its mechanism of action, ZYNLONTA® can cause embryo-fetal harm when administered to a pregnant woman because it contains a genotoxic compound (SG3199) and affects actively dividing cells.
Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with ZYNLONTA® and for 10 months after the last dose. Advise male patients with female partners of reproductive potential to use effective contraception during treatment with ZYNLONTA® and for 7 months after the last dose.
ADVERSE REACTIONS
In a pooled safety population of 215 patients (Phase 1 and LOTIS-2), the most common (>20%) adverse reactions, including laboratory abnormalities, were thrombocytopenia, increased gamma- glutamyltransferase, neutropenia, anemia, hyperglycemia, transaminase elevation, fatigue, hypoalbuminemia, rash, edema, nausea, and musculoskeletal pain.
In LOTIS-2, serious adverse reactions occurred in 28% of patients receiving ZYNLONTA®. The most common serious adverse reactions that occurred in ≥2% receiving ZYNLONTA® were febrile neutropenia, pneumonia, edema, pleural effusion, and sepsis. Fatal adverse reactions occurred in 1%, due to infection.
Permanent treatment discontinuation due to an adverse reaction of ZYNLONTA® occurred in 19% of patients. Adverse reactions resulting in permanent discontinuation of ZYNLONTA® in ≥2% were gamma- glutamyltransferase increased, edema, and effusion.
Dose reductions due to an adverse reaction of ZYNLONTA® occurred in 8% of patients. Adverse reactions resulting in dose reduction of ZYNLONTA® in ≥4% was gamma-glutamyltransferase increased.
Dosage interruptions due to an adverse reaction occurred in 49% of patients receiving ZYNLONTA®. Adverse reactions leading to interruption of ZYNLONTA® in ≥5% were gamma-glutamyltransferase increased, neutropenia, thrombocytopenia, and edema.
DOSAGE MODIFICATIONS AND DELAYS
Recommended Dosage Modifications for Adverse Reactions
For any Grade 3 or greater nonhematologic toxicity, ZYNLONTA® should be held until the toxicity resolves to Grade 1 or less. For neutropenia: if absolute neutrophil count is <1 x 109/L, withhold ZYNLONTA® until the neutrophil count returns to 1 x 109/L or higher. For thrombocytopenia: if platelet count is <50,000/mcL, withhold ZYNLONTA® until the platelet count returns to 50,000/mcL or higher. For Grade 2 or greater edema or effusion, ZYNLONTA® should be held until the toxicity resolves to Grade 1 or less.
Recommendations for Dosage Delays
If dosing is delayed by more than 3 weeks due to toxicity related to ZYNLONTA®, reduce subsequent doses by 50%. If toxicity reoccurs following dose reduction, consider discontinuation. Note: If toxicity requires dose reduction following the second dose of 0.15 mg/kg (C2D1), the patient should receive the dose of 0.075 mg/kg for Cycle 3.
You may report side effects to the FDA at (800) FDA-1088 or www.fda.gov/medwatch. You may also report side effects to ADC Therapeutics at 1-855-690-0340.
Please see the full Prescribing Information for additional Important Safety Information.
Reference: 1. ZYNLONTA® Prescribing Information. ADC Therapeutics SA; 2022. 2. Caimi PF, Ai WZ, Alderuccio JP, et al. Long-term responses with loncastuximab tesirine: updated results from LOTIS-2, the pivotal phase 2 study of patients with relapsed/refractory diffuse large B-cell lymphoma. Poster presented at: European Hematology Association 2023 Hybrid Congress. Frankfurt, Germany and virtual, June 8-11, 2023.